
The 3-Hit model: Our work on the cell danger response (CDR)1-11 led to the development of a 3-hit model of autism spectrum disorder [pdf]. In this model, ASD results from the sequential interaction of 3 factors: (1) DNA changes that sensitize mitochondrial metabolism, intracellular calcium handling, and ATP-related purinergic signaling to environmental change; (2) early prenatal or postnatal activation of the CDR by environmental factors like infection, immune dysregulation, metabolic disturbance, or toxicant exposure; and (3) prolonged or recurrent exposure to CDR-activating triggers for 3-6 months from the late 1 st trimester to 18-36 months of age. When mitochondrial, metabolic, and/or immune resources are usurped for too long during this critical window of neurodevelopment, the risk of ASD and several commonly co-occurring medical conditions is increased.
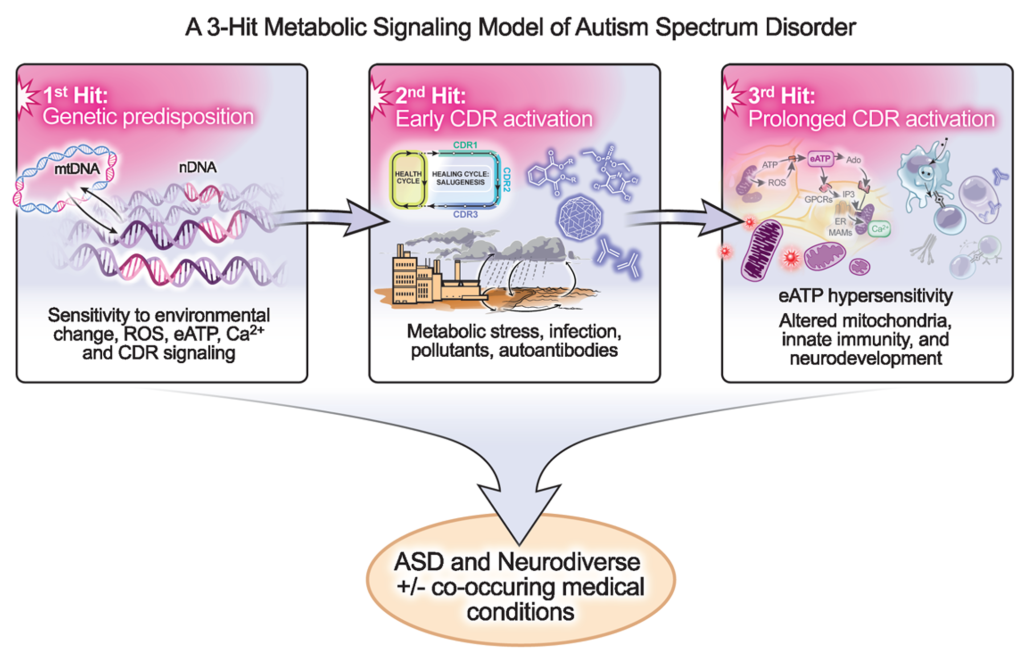
The 3-hit model integrates over 100 years of autism research. It doesn’t just change the way we think about ASD. It changes what we can do about it. Because the 2nd and 3rd hits are modifiable, the new model predicts that if CDR-activating air and water pollutants are decreased, new prenatal and newborn screening methods are used to identify the most vulnerable children, co-occurring medical conditions are treated, and new supports for metabolic and neuroimmune resilience are given to those children at increased risk, that like phenylketonuria (PKU)12 , the most disabling symptoms of ASD can be prevented before they occur in some children, and decreased in others.
Once the symptoms of ASD have occurred, the model also predicts that new antipurinergic treatment trials of drugs like suramin can point the way to decreasing the most disabling symptoms in children and adults. Suramin is just the first in a new class of medicines that has not yet been developed. It is a steppingstone. It is not the final answer. If larger suramin trials are successful, many new medicines will be designed to calm the hypersensitive ATP signaling networks that cause the CDR to persist in ASD. These new medicines may one day help some children come off spectrum and reduce ASD’s most disabling symptoms in others.
- The 3-hit model of ASD [pdf, news report]
- An in-depth review of antipurinergic therapy for autism
- The SAT1 clinical trial, published in Annals of Clinical and Translational Neurology
- Parent statements
- Questions and answers with Dr. Naviaux
- Cautionary statement about suramin
- A physician’s poem of hope
- UCSD website with content about the suramin clinical trial
- N of One website with content about the trial
- UCSD Triton Magazine Story, Fall 2017
- Suramin updates, 2020—An Interview with Dr Naviaux by Lisa Ackerman
- Reading—Purines, the CDR, ASD, and Healing
References
- Naviaux, R.K. A 3-hit metabolic signaling model for the core symptoms of autism spectrum disorder. Mitochondrion, 102096. 2025.
- Naviaux, R.K. Antipurinergic therapy for autism—an in-depth review. Mitochondrion. PMID: 29253638, 2017.
- Naviaux, J.C., et al. Antipurinergic therapy corrects the autism-like features in the Fragile X (Fmr1 knockout) mouse model. Molecular Autism 6, 1 (2015).
- Naviaux, R.K., et al. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Translational Psychiatry. PMID: 24937094, 2014.
- Naviaux, R.K. Metabolic features of the cell danger response. Mitochondrion 16, 7-17 (2014).
- Naviaux, R.K., et al. Antipurinergic Therapy Corrects the Autism-Like Features in the Poly(IC) Mouse Model. PloS One (2013).
- Naviaux, R.K. Oxidative shielding or oxidative stress? J Pharmacol Exp Ther 342, 608-618 (2012).
- Naviaux, R.K. Mitochondria and Autism. in The Neuroscience of Autism Spectrum Disorders (eds. Buxbaum, J.D. & Hof, P.R.) 179-193 (Academic Press, Elsevier, Waltham, MA, 2012).
- Burnstock, G. Introduction to purinergic signalling in the brain. Advances in experimental medicine and biology 986, 1-12 (2013).
- Burnstock, G. Purinergic mechanisms and pain–an update. Eur J Pharmacol 716, 24-40 (2013).
- Burnstock, G. Discovery of purinergic signalling, the initial resistance and current explosion of interest. British journal of pharmacology 167, 238-255 (2012).
- van Spronsen FJ, Blau N, Harding C, Burlina A, Longo N, Bosch AM. Phenylketonuria. Nat Rev Dis Primers 2021; 7(1): 36. PMID: 34017006


